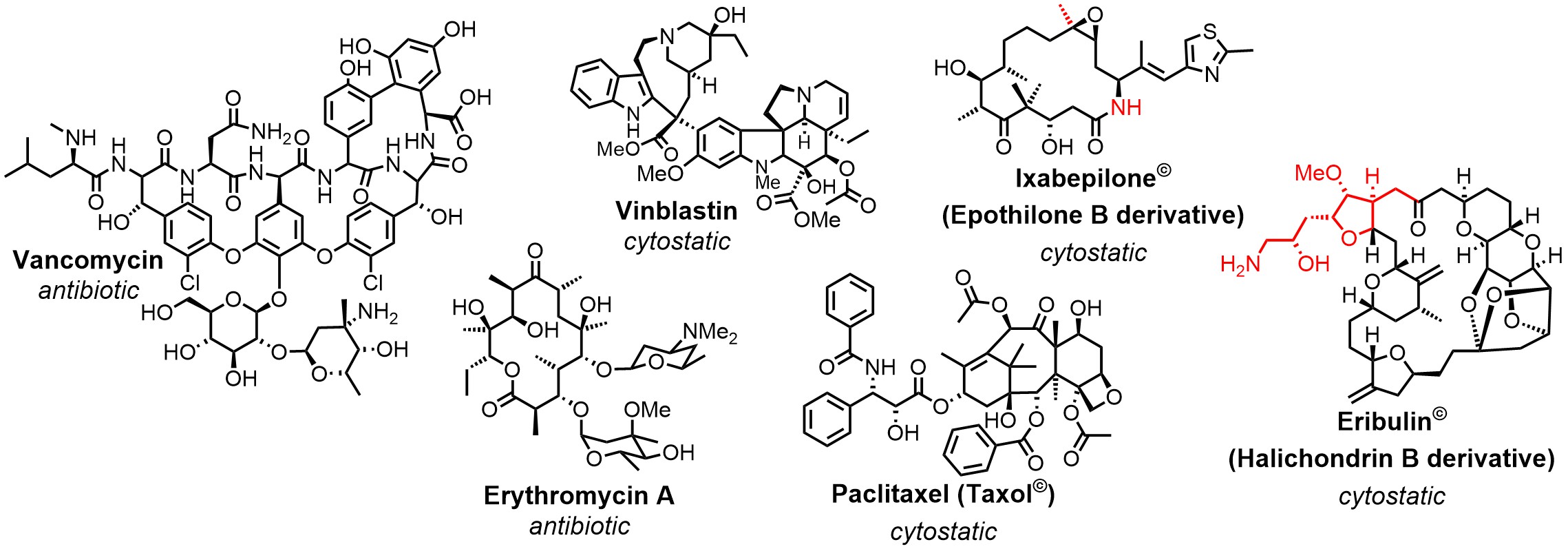
Natural Products And Total Synthesis
Natural products play an important role in drug discovery. More than 60% of all newly approved drugs between 1981 and 2014 derive either directly from them or are highly inspired by their structures.[1] The evolutionary privileged structures of natural products make them superior leads, starting from which the probability to find drug candidates is much higher than from non-natural compounds. Structure activity relationship (SAR) studies are an integral part of drug development and represent a particular challenge for complex natural products. The efficient production of derivative libraries is a bottleneck that often limits the full exploitation of the pharmacological potential of natural products.
One mayor objective is thus improving the available methodology towards greater efficiency, while at the same time retaining maximum flexibility in the choice of the target structure. Total synthesis has taken an impressive development during the past decades leading to the situation that, in principle, any desired target molecule is accessible by chemical means. The development of new reactions (e.g. Olefin cross metathesis), synthetic concepts (e.g. Diversity-oriented synthesis) and technologies (e.g. Flow chemistry) has also led to a successive expansion of the efficiency and scope of total chemical synthesis. This ongoing process is complemented by hybrid approaches that merge chemical methodology with tools from other fields of the natural sciences, such as biotechnology. Thanks to its unmatched flexibility, chemical synthesis will remain a key technology in the field of natural products.
[1] D. J. Newman, G. M. Cragg, J. Nat. Prod. 2016, 79, 629–661.
Our research interests in this field
One of the aims of research in the Hahn group is the synthesis of biologically active polyketide natural products with potential for drug development. A tool we extensively rely on is enzymatic transformations (chemoenzymatic synthesis). These can simplify synthetic routes and make them significantly more resource-efficient, thus facilitating the synthesis of complex libraries of derivatives.
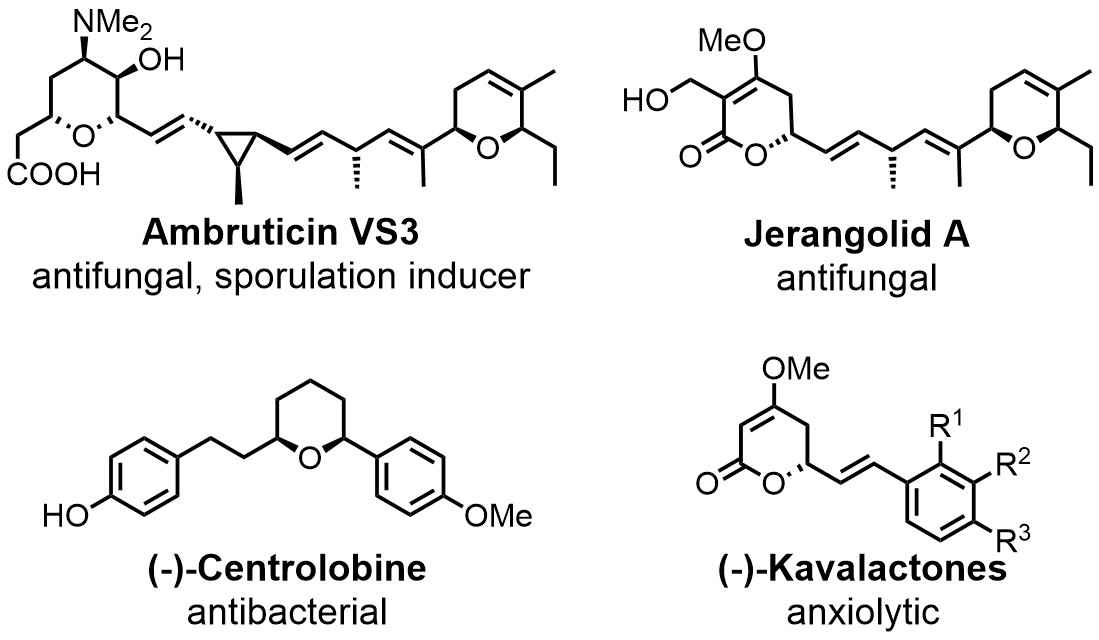
Chemoenzymatic total syntesis of the ambruticins and jerangolids is a long-term project in the group. Newly characterised enzymes are also applied in syntheses of (-)-centrolobine, the (-)-kavalactones and others.
We have a long-term interest in the ambruticins and the jerangolids.[2] These polyketides have been isolated from myxobacteria in the 1970ies and early 1990ies, respectively. Their unique biological activity profile, which among others combines significant antifungal activity with low toxicity towards mammals, has attracted the interest of academia as well as industry. Successful total syntheses have previously been developed for both compounds. We are working to further improve the synthetic approach by integrating efficient transformations with key biosynthetic enzymes, ultimately providing additional room for the synthesis of derivatives. We also apply this approach to a couple of other target molecules (see Figure on the left).
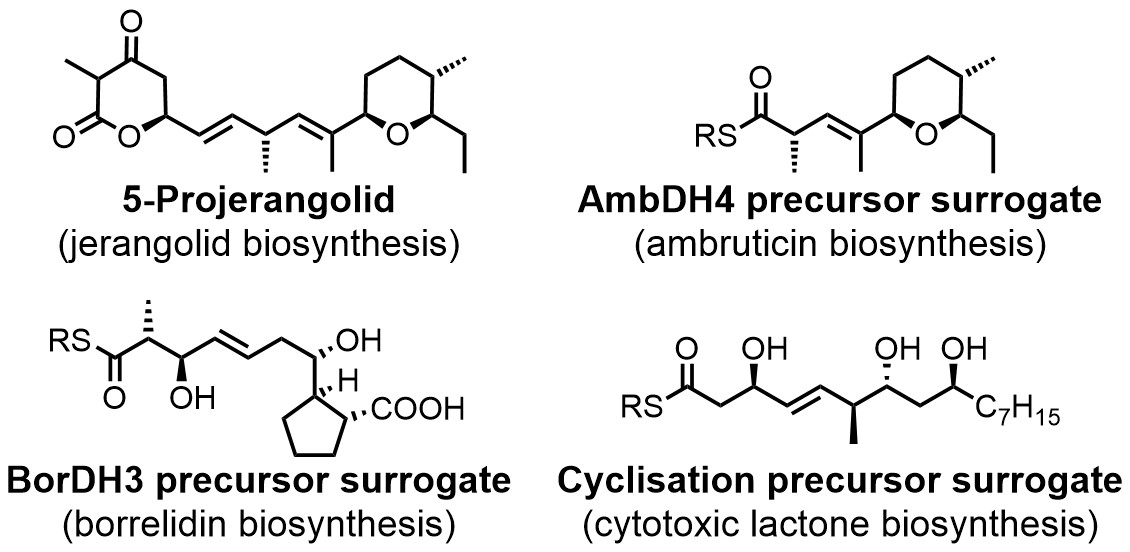
Compounds that were prepared for enzyme characterisation projects by chemical total synthesis. DH: dehydratase.[3-5]
Besides our studies on the total synthesis of natural products, we also pursue projects on the synthesis of biosynthetic intermediates and their surrogates. These compounds are often indispensable for the biochemical characterisation of new biosynthetic enzymes and thus also for the development of chemoenzymatic total syntheses.
Such target molecules can exhibit a degree of complexity that puts their preparation on a level with the total syntheses of genuine natural products and they also often exhibit additional sensitivities. This requires creative solutions for their application in enzymatic assays and the development of new synthetic methods for their making. In recent years, we have developed multiple syntheses of complex intermediates for studies of various biosynthetic pathways and chemoenzymatic total syntheses (see Figure on the left).
[2] F. Hahn, F. Guth, Nat. Prod. Rep. 2020, 37, 1300–1315.
[3] F. Lindner, S. Friedrich, F. Hahn J. Org. Chem. 2018, 22, 14091–14101.
[4] G. Berkhan, C. Merten, C. Holec, F. Hahn, Angew. Chem. Int. Ed. 2016, 43, 13589–13592.
[5] F. Hahn, N. Kandziora, S. Friedrich, P. F. Leadlay, Beilstein. J. Org. Chem. 2014, 10, 634–640.
[6] S. Derra, L. Schlotte, F. Hahn, Chemistry 2023, 5 (2), 1407-1418.
[7] S. Derra, J. Hoffmann, F. Hahn, Synlett 2023, doi: 10.1055/a-2216-4521.